Benefits

FDA Listed and Registered
Clinically Tested and Proven
- Soothing, moisturizing formula with faint citrus scent
- Convenient and easy to use
- 12-hour persistence
Our Nozin Products
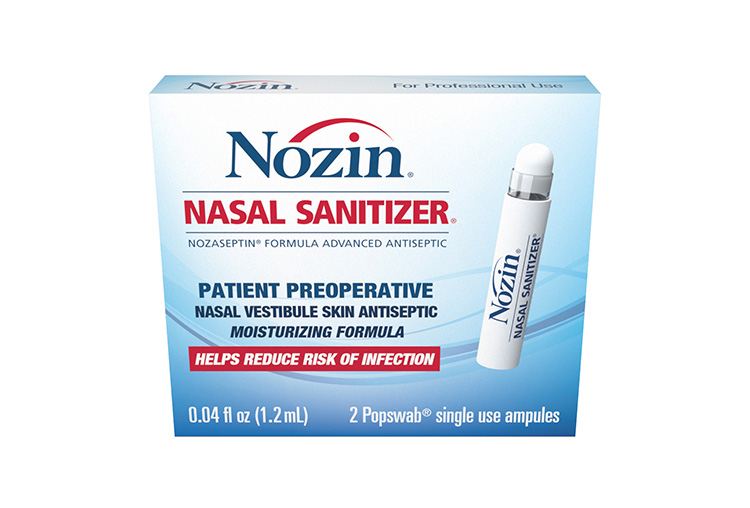
Nozin Preoperative Pack
Nozin Preoperative Pack
- Kills 99.99% of germs
- Clinically proven
- Moisturizing formula
- 12-hour persistence
- Helps reduce risk of infection
- Decreases nasal bacteria carriage
Nozin Cardboard Bin Ampules
Nozin Cardboard Bin Ampules
- Kills 99.99% of germs
- Clinically proven
- Moisturizing formula
- 12-hour persistence
- Helps reduce risk of infection
- Decreases nasal bacteria carriage
Nozin Blister Pack Ampules
Nozin Blister Pack Ampules
- Kills 99.99% of germs
- Clinically proven
- Moisturizing formula
- 12-hour persistence
- Helps reduce risk of infection
- Decreases nasal bacteria carriage
Nozin 12ml Bottle
Nozin 12ml Bottle
- Kills 99.99% of germs
- Clinically proven
- Moisturizing formula
- 12-hour persistence
- Helps reduce risk of infection
- Decreases nasal bacteria carriage